Penn State advances pediatric cancer research through new targeted therapy collaboration
Penn State College of Medicine is advancing its commitment to cutting-edge cancer research and early-phase clinical trials through a new collaboration aimed at accelerating a promising targeted therapy for pediatric brain[...]
Penn State’s Beat Childhood Cancer Research Consortium welcomes SJD Pediatric Cancer Center Barcelona, strengthening global leadership in pediatric cancer research
The Pediatric Cancer Center Barcelona (PCCB) at Hospital Sant Joan de Déu has become the first center outside North America to join the Beat Childhood Cancer (BCC) Research Consortium, an international pediatric cancer research[...]
Penn State College of Medicine, Beat Childhood Cancer Research Consortium and Four Diamonds launch clinical trial that aims to combat solid tumors
In a step toward fighting solid tumors in children and young adults, Penn State College of Medicine, the Beat Childhood Cancer Research Consortium and Four Diamonds, along with Senhwa Biosciences, Inc. are launching a pioneering clinical[...]
Q&A: Finding novel therapies for childhood cancer
Since the first time she treated a child with neuroblastoma, Giselle Saulnier Sholler wanted to do the impossible for her patients. Neuroblastoma is the most common extracranial solid tumor in children with[...]
FDA approves pediatric neuroblastoma drug based on Penn State professor’s work
Giselle Saulnier Sholler, professor of pediatrics and of pharmacology, reflects on 20 years working to change cancer outcomes for children HERSHEY, Pa. — In 2003, the first year of her[...]
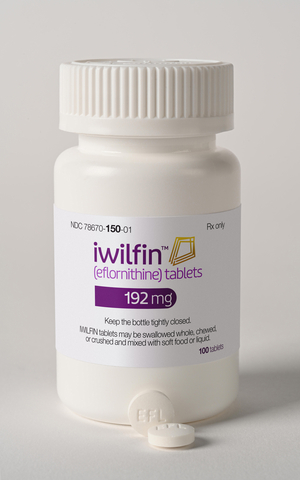
US WorldMeds Announces FDA Approval of IWILFIN™ (eflornithine) to Strengthen Fight Against Aggressive Childhood Cancer
LOUISVILLE, Ky.--(BUSINESS WIRE)--USWM, LLC (US WorldMeds) today announced that the U.S. Food and Drug Administration (FDA) has approved IWILFIN™ (eflornithine) 192 mg tablets, a groundbreaking oral maintenance therapy for high-risk[...]
Penn State Health recruits leading researcher to fight pediatric cancer
Cris Collingwood//August 2, 2023 An internationally known physician-scientist and her research consortium have joined Penn State Health to continue efforts to eradicate pediatric cancer. Penn State Health and Penn State College of Medicine[...]
The Power of Will
Will Lacey was just a baby when doctors diagnosed a rare form of cancer and told his family there was only one end. Nobody then could imagine the journey ahead,[...]
Michigan Child Trials Target Genetic Roots of Cancers
Thank you Detroit News for featuring a story about the work we are doing. It wouldn’t be possible without the support of Dell, TGen Foundation and our generous community. Together,[...]
Feasibility of Implementing Molecular-guided Therapy for the Treatment of Patients With Relapsed or Refractory Neuroblastoma
DOWNLOAD THE FULL ARTICLE (PDF).